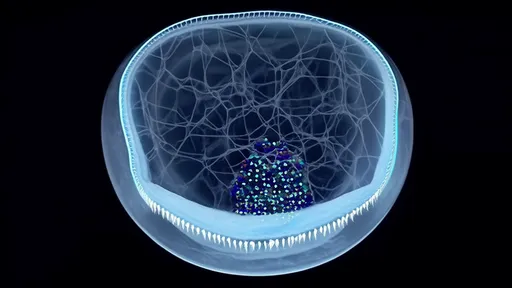
In a groundbreaking development that could revolutionize corneal transplantation, scientists have successfully developed artificial corneas using genetically modified spider silk proteins produced by silkworms. This innovative biomaterial, which mimics the optical clarity and mechanical strength of natural human corneas, offers new hope for millions suffering from corneal blindness worldwide.
The research team, led by bioengineers at the Karolinska Institute in Sweden, spent nearly a decade perfecting the technique to produce recombinant spider silk proteins in transgenic silkworms. Unlike traditional spider silk farming which requires maintaining large numbers of spiders, this method leverages the established sericulture industry while overcoming the challenges of spider territorial behavior and cannibalism.
What makes this artificial cornea remarkable is its unique combination of properties. The silk protein scaffold demonstrates exceptional transparency, allowing over 95% of visible light to pass through - comparable to natural corneal tissue. More importantly, the material shows outstanding biocompatibility in preliminary animal trials, with minimal immune rejection observed in test subjects.
The manufacturing process begins with identifying and isolating the specific spider silk genes responsible for producing the strongest and most elastic silk varieties. These genes are then inserted into silkworm embryos using CRISPR-Cas9 gene editing technology. The resulting transgenic silkworms spin cocoons containing the hybrid silk proteins, which are subsequently harvested and purified.
Dr. Helena Johansson, the project's lead researcher, explains: "The beauty of this system lies in its scalability. Silkworms have been domesticated for thousands of years and can be farmed efficiently. By making them produce spider silk proteins, we've essentially created miniature bioreactors that manufacture high-quality corneal biomaterial at low cost."
Current corneal transplants rely on donor tissue, which remains in critically short supply globally. The World Health Organization estimates that 12.7 million people await corneal transplants, with less than 1 in 70 receiving the needed surgery. This artificial cornea could potentially eliminate both the donor shortage and the risk of disease transmission associated with human tissue transplants.
Beyond addressing the supply issue, the silk protein cornea offers several technical advantages. Its porous structure allows for better integration with host tissue compared to synthetic polymer alternatives. The material also demonstrates superior resistance to enzymatic degradation in the eye environment, maintaining structural integrity for extended periods.
Clinical trials are expected to begin within two years, pending regulatory approvals. If successful, this technology could be adapted for other ocular applications, including drug delivery systems and retinal repair scaffolds. The research team has already filed multiple patents covering both the transgenic silkworm technology and the specialized processing methods for the silk proteins.
While challenges remain in perfecting the surgical implantation techniques and ensuring long-term performance, the scientific community has greeted this development with enthusiasm. Many experts believe this represents the most promising advance in corneal replacement technology since the first successful human transplant was performed in 1905.
The implications extend beyond ophthalmology. The success of producing complex spider silk proteins in silkworms opens new possibilities for biomaterial production. Similar approaches could potentially be applied to create other medical-grade materials, from artificial tendons to advanced wound dressings, all derived from nature's original high-performance fibers.
As research continues, the team is working to optimize the protein composition for different patient needs. Variations in silk protein blends could allow customization of the artificial corneas for specific optical properties or mechanical requirements, potentially offering better outcomes than standardized donor tissue.
This breakthrough exemplifies the growing field of bioinspired materials, where scientists look to nature's solutions to solve complex medical challenges. By combining ancient sericulture practices with cutting-edge genetic engineering, researchers have created a sustainable, scalable solution to one of medicine's most persistent shortages.
The economic impact could be significant as well. Current corneal transplant procedures cost thousands of dollars in developed nations and remain largely inaccessible in poorer regions. The silk protein corneas, once mass-produced, could potentially reduce costs by 80% or more, making vision-restoring surgery available to millions who currently have no hope of treatment.
Looking ahead, researchers anticipate that further refinements could lead to even more advanced versions of the technology. Some speculate about incorporating growth factors or anti-scarring agents directly into the silk matrix, or creating "smart" corneas that could monitor intraocular pressure or other health indicators.
For now, the focus remains on bringing this first-generation artificial cornea to market. With continued progress, the day may soon come when blindness caused by corneal damage becomes as treatable as routine cataract surgery - all thanks to the unlikely combination of spider silk proteins and genetically modified silkworms.

By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025
By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025